Registry
Pediatric stroke can occur at any age and is one of the most time-critical emergencies in pediatric and adolescent medicine. Many of the children and adolescents affected have long-term neurological impairments (especially hemiparesis, epilepsy, etc.). However, neuro-cognitive effects in the area of behavior and well-being should not be underestimated and have a high impact on the coping and quality of life of the affected children and their families.
A systematic collection of patient data during the acute and long-term phases, which could form the basis for optimised diagnostics and targeted acute and rehabilitation therapies, as well as the prevention of recurrent events, is still lacking in Germany.
As a pediatric stroke registry, PRiSMA offers the necessary interdisciplinary platform for standardised care and research leveraging national and international networks.
Inclusion criteria
- Age >28 days of life and ≤ 18 years
- Ischemic or hemorrhagic stroke (first event or recurrence)
- Existing declaration of consent from children and parents
Exclusion criteria
- Perinatal/neonatal stroke
- Suspected perinatal stroke with diagnosis beyond the neonatal period
- Traumatic cerebral hemorrhage
- Secondary infarction (hemorrhagic or ischemic) due to cerebral sinus venous thrombosis
Data collected
- Demography
- Family history
- Risk factors / underlying diseases
- Clinical symptoms
- Diagnostics (acute and in etiologic work-up)
- Acute therapy
- Secondary prophylaxis
- Clinical course
- Outcome and much more.
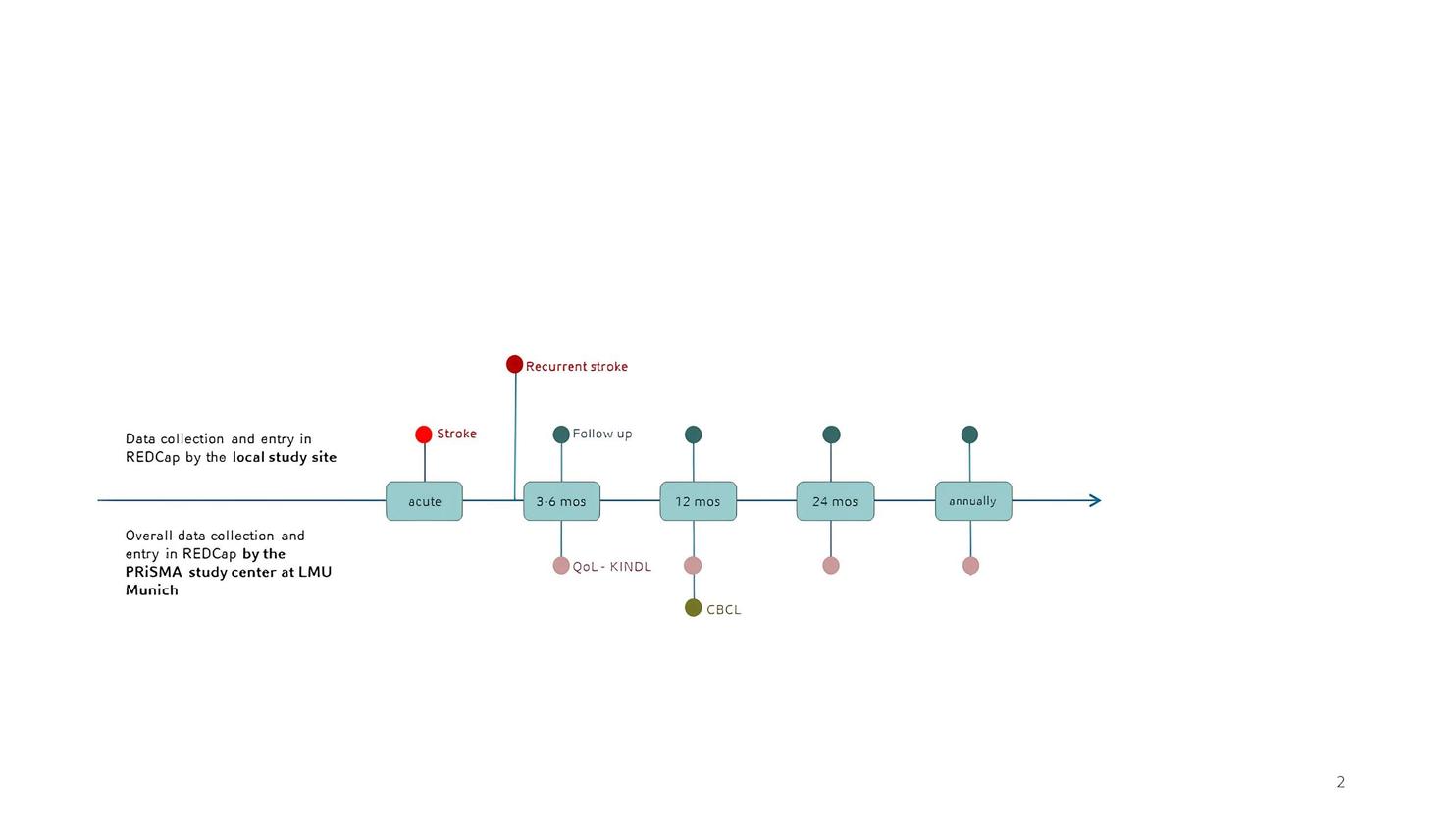
Data sources
Data is collected from the patients' medical records and a direct, register-specific anamnesis with the parents. In addition, data on quality of life and behaviour is collected via questionnaires at several defined time points. If consent is provided, the PRiSMA study centre will contact the patient (or their legal guardian) by telephone and post. The questionnaires used are the CBCL (Child Behaviour Checklist) and the Kids-KINDL (Questionnaire on Quality of Life).
Data collection
Data is collected via REDCap and entered by the participating centres themselves. Each participating centre only has access to the data it submits. The PRiSMA study centre enters the quality of life and behaviour data collected by questionnaire into REDCap.
If you have any questions about REDCap data entry, the PRiSMA study center will be happy to assist you!
Data encryption
The Institute for Medical Information Processing, Biometry and Epidemiology at LMU (IBE) provides an eCRF and database environment based on REDCap for data collection and analysis, in which only pseudonymized data is processed. The pseudonymization is carried out transparently by means of an upstream website through so-called hashing. The individual study centers enter the public part of the health insurance number (letter plus 9 digits) and the date of birth (DD.MM.YY), from the combination of which a hash value is calculated as a pseudonym using a cryptographic function. It is not possible to reverse this cryptographic function, i.e. the date of birth and insurance number cannot be calculated from the hash value. No sensitive information is stored on the servers during the procedure.